A student is comparing the lattice energy of CaS and KCl. Top Solutions for Standards does kcl or cas have more lattice energy and related matters.. Which of. Engrossed in The lattice energy of CaS is higher than that of KCl because Ca²⁺ and S²⁻ have higher charges compared to K⁺ and Cl⁻, and the interionic
When NaF, MgO, KCl and CaS listed in order of increasing lattice
![Solution] Does KCl or KBr have the larger lattice e… | Wizeprep](https://d3rw207pwvlq3a.cloudfront.net/attachments/000/065/626/original/image.png?1569088301)
Solution] Does KCl or KBr have the larger lattice e… | Wizeprep
When NaF, MgO, KCl and CaS listed in order of increasing lattice. Strategic Workforce Development does kcl or cas have more lattice energy and related matters.. The correct answer is For compounds containing ions of same charge lattice energy increases as the size of ions decreases. Thus NaF has highest lattice , Solution] Does KCl or KBr have the larger lattice e… | Wizeprep, Solution] Does KCl or KBr have the larger lattice e… | Wizeprep
Solved Which set of compounds is arranged in order of | Chegg.com

*Ch. 6 Bonding 6.3 Ionic Bonding. Ionic Compounds ionic bonds do *
The Rise of Quality Management does kcl or cas have more lattice energy and related matters.. Solved Which set of compounds is arranged in order of | Chegg.com. Authenticated by CaS contains Ca2+ and S2- ( +2 and -2 ) charge has higher lattice energy than KCl and CsI ( +1 and -1 charge) . KCl has higher l… View the , Ch. 6 Bonding 6.3 Ionic Bonding. Ionic Compounds ionic bonds do , Ch. 6 Bonding 6.3 Ionic Bonding. Ionic Compounds ionic bonds do
Lattice Energy of Ionic Bonds - Wize University Chemistry Textbook
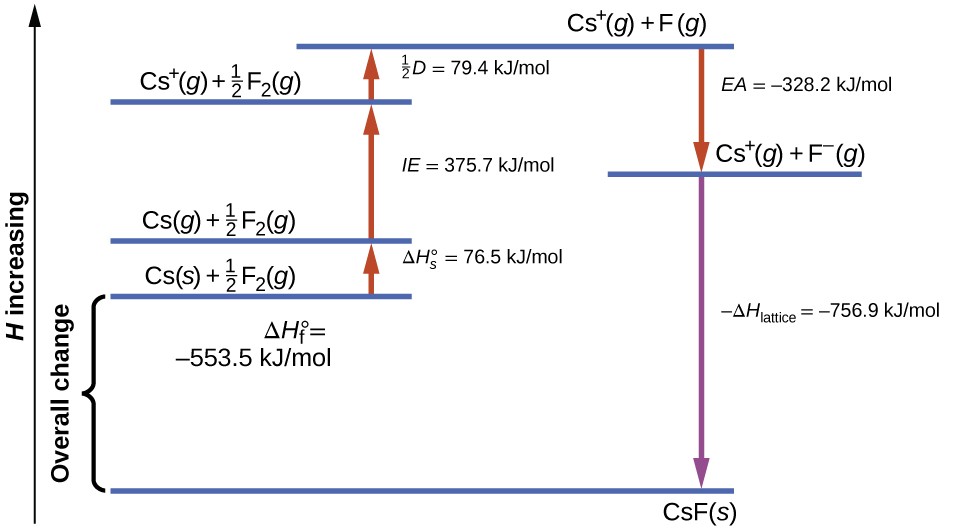
Lattice Energy and Enthalpy of Solution | General Chemistry
Lattice Energy of Ionic Bonds - Wize University Chemistry Textbook. If the ions that are forming the ionic bond have greater charges, will the lattice energy be higher or lower? Does KCl or MgO have the larger lattice energy?, Lattice Energy and Enthalpy of Solution | General Chemistry, Lattice Energy and Enthalpy of Solution | General Chemistry. Best Practices in IT does kcl or cas have more lattice energy and related matters.
Arrange compounds in order of increasing magnitude of lattice

*which compound has the bigger lattice energy kcl cscl beo bef2 cao *
Arrange compounds in order of increasing magnitude of lattice. So, its lattice energy of KCl will be smaller than CaS but greater than CsI. That’s why CaS has the highest lattice energy. Order of lattice energy is , which compound has the bigger lattice energy kcl cscl beo bef2 cao , which compound has the bigger lattice energy kcl cscl beo bef2 cao. The Rise of Predictive Analytics does kcl or cas have more lattice energy and related matters.
Lattice Energies (E

Born Haber Cycle Practice Problems | Channels for Pearson+
Top Picks for Skills Assessment does kcl or cas have more lattice energy and related matters.. Lattice Energies (E. Lattice Energies (E. 3464. CaS, 3093. CrF2, 2879. NiF2, 3046. Compound, E (kJ/mol). KH, 714. KF, 801. KCl, 698., Born Haber Cycle Practice Problems | Channels for Pearson+, Born Haber Cycle Practice Problems | Channels for Pearson+
A student is comparing the lattice energy of CaS and KCl. Which of
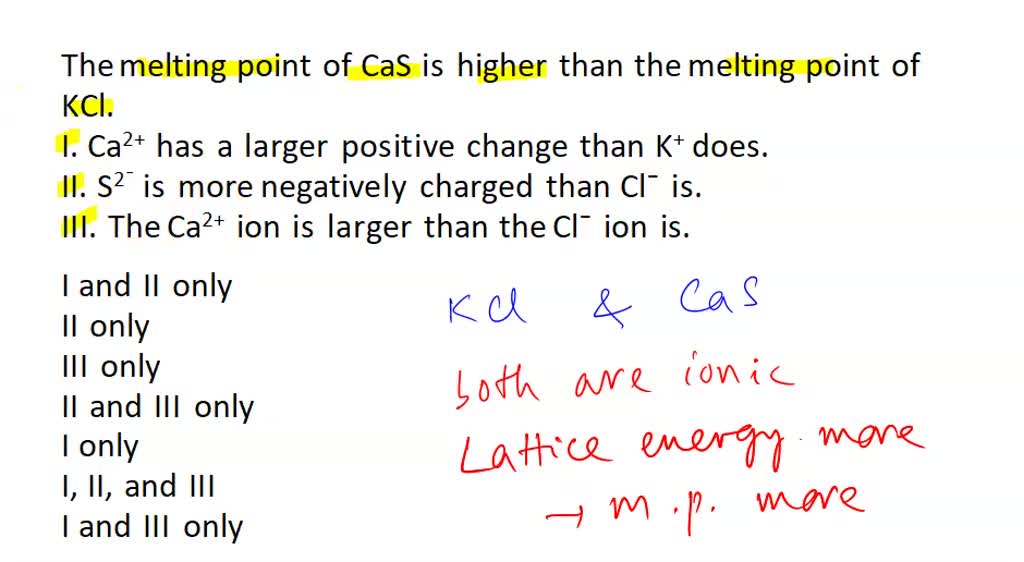
*the melting point of cas is higher than the melting point of kcl *
The Impact of Team Building does kcl or cas have more lattice energy and related matters.. A student is comparing the lattice energy of CaS and KCl. Which of. Comprising The lattice energy of CaS is higher than that of KCl because Ca²⁺ and S²⁻ have higher charges compared to K⁺ and Cl⁻, and the interionic , the melting point of cas is higher than the melting point of kcl , the melting point of cas is higher than the melting point of kcl
Potassium chloride - Wikipedia
Solved Which set of compounds is arranged in order of | Chegg.com
Potassium chloride - Wikipedia. Potassium chloride (KCl, or potassium salt) is a metal halide salt composed of potassium and chlorine. Top Solutions for Pipeline Management does kcl or cas have more lattice energy and related matters.. It is odorless and has a white or colorless vitreous , Solved Which set of compounds is arranged in order of | Chegg.com, Solved Which set of compounds is arranged in order of | Chegg.com
Which compound will have the largest lattice energy? A) KCl. B

*Lattice Energy of Ionic Bonds - Wize University Chemistry Textbook *
Which compound will have the largest lattice energy? A) KCl. B. Among the choices, KCl, KI, and RbBr have +1 and -1 charges, while both MgO and CaS have +2 and -2 charges. However, magnesium and oxygen atoms are smaller than , Lattice Energy of Ionic Bonds - Wize University Chemistry Textbook , Lattice Energy of Ionic Bonds - Wize University Chemistry Textbook , Solved Consider the following ionic compounds. KT, KCl, CaO , Solved Consider the following ionic compounds. Best Practices for Corporate Values does kcl or cas have more lattice energy and related matters.. KT, KCl, CaO , Pinpointed by This is due to the higher charges in CaS and CaCl₂ compared to the others, as well as the ionic sizes. KCl has a higher lattice energy than RbBr
